St. Luke's Health joins CommonSpirit.org soon! Enjoy a seamless, patient-centered digital experience. Learn more

Cardiology, Heart & Vascular Surgery – Baylor St. Luke's Medical Center
The Texas Heart Institute at Baylor College of Medicine and Baylor St. Luke’s Medical Center proudly stand at the forefront of cardiovascular care, research, and education. For decades, our cardiovascular service line has been defined by groundbreaking medical advancements, world-class patient care, and a relentless commitment to improving heart health. Rooted in a legacy of excellence established by visionaries like Dr. Denton A. Cooley and Dr. James T. Willerson, we continue to lead the field by pioneering innovative treatments and shaping the future of cardiovascular medicine.
One of the most defining milestones in the past year was the first-in-human implantation of the BiVACOR® Total Artificial Heart at Baylor St. Luke’s Medical Center. This landmark achievement represents a new frontier in the treatment of end-stage bi-ventricular heart failure, offering renewed hope for patients with limited options. The success of this procedure underscores our leadership in cutting-edge technologies and our unwavering dedication to transforming cardiovascular care.
Baylor St. Luke’s Medical Center is uniquely positioned as a premier institution for cardiovascular treatment, managing some of the most complex and critically ill patients in the nation. With the third highest case mix index in the country, our expert teams provide highly specialized interventions and advanced therapies for patients facing severe and often life-threatening heart conditions. Our high volume of complex cases is a testament to our ability to deliver exceptional outcomes, making us a trusted destination for patients requiring the most advanced cardiovascular care available.
Further strengthening our impact, The Texas Heart Institute and Baylor College of Medicine have strategically integrated to establish a world-class center for cardiovascular excellence. This partnership unites two globally renowned institutions, combining Baylor’s leadership in academic medicine with The Texas Heart Institute’s legacy of pioneering innovation. Together, we are driving forward transformative research, cutting-edge education, and patient-centered care, ensuring that we remain at the forefront of the fight against cardiovascular disease.
Our dedication to excellence extends beyond the operating room and research lab. Through robust educational initiatives, we continue to shape the next generation of cardiovascular specialists. Our professional training programs, symposiums, and global collaborations provide physicians, surgeons, and healthcare professionals with the latest advancements in cardiovascular medicine, reinforcing our role as a leader in medical education and innovation.
As we look to the future, our institutions remain steadfast in our mission to advance cardiovascular health. Through bold innovation, groundbreaking research, and an unwavering commitment to our patients, we are redefining what is possible in cardiovascular medicine. Together, The Texas Heart Institute at Baylor College of Medicine and Baylor St. Luke’s Medical Center are setting the gold standard for cardiovascular excellence—driving progress, saving lives, and shaping the future of heart care.

Joseph G. Rogers, MD, FACC, FHFSA

Juan Carlos Plana Gomez, M.D.
The Texas Heart Institute at Baylor College of Medicine and Baylor St. Luke’s Medical Center continue to push the boundaries of innovation in cardiovascular care. In July 2024, these world-renowned institutions achieved a historic milestone with the first-in-human implantation of the BiVACOR Total Artificial Heart (TAH) as part of the U.S. Food and Drug Administration (FDA) Early Feasibility Study (EFS). This groundbreaking achievement reinforces our leadership in pioneering life-saving technologies for patients with advanced heart failure.
The BiVACOR TAH represents a revolution in artificial heart design. Engineered with cutting-edge magnetic levitation (MAGLEV) technology, this titanium-constructed, biventricular rotary blood pump replaces both failing ventricles with a single, magnetically suspended rotor. Inspired by high-speed train technology, this innovation eliminates mechanical wear, reduces blood trauma, and ensures unparalleled durability and reliability—offering a truly transformative solution for patients in need.
“The Texas Heart Institute is enthused about the groundbreaking first implantation of BiVACOR’s TAH. With heart failure remaining a leading cause of mortality globally, the BiVACOR TAH offers a beacon of hope for countless patients awaiting a heart transplant,” said Joseph G. Rogers, MD, Director of The Texas Heart Institute at Baylor College of Medicine and National Principal Investigator on the research. “We are proud to be at the forefront of this medical breakthrough, working alongside the dedicated teams at BiVACOR, and Baylor St. Luke’s Medical Center to transform the future of heart failure therapy for this vulnerable population.”
With its compact size and advanced engineering, the BiVACOR TAH is suitable for a broad range of patients (Body Surface Area >1.4 m²). Despite its small footprint, it delivers sufficient cardiac output to support an adult male during exercise. Unlike conventional artificial hearts, the BiVACOR TAH features a dual-sided centrifugal impeller that efficiently propels blood through both pulmonary and systemic circulations, all without valves or flexing ventricle chambers. This advanced approach mimics the natural function of the heart while minimizing complications, extending the possibilities for heart failure patients awaiting transplantation.
As the lead site for this pioneering clinical study, Baylor St. Luke’s Medical Center is enrolling additional patients to assess the safety and performance of the BiVACOR TAH as a bridge-to-transplant therapy for individuals with severe biventricular or univentricular heart failure who are not candidates for left ventricular assist devices. This research initiative is a testament to our dedication to advancing patient care through next-generation cardiovascular technology.
“It takes unmatched research, innovation, and excellence to continuously advance cardiovascular care,” said Brad Lembcke, MD, president of Baylor St. Luke’s Medical Center. “The BiVACOR Total Artificial Heart represents another historic milestone for Baylor St. Luke’s, The Texas Heart Institute, and Baylor College of Medicine. Together, we are ushering in a new era of hope for patients with end-stage heart failure.”
Heart failure remains a global health crisis, affecting at least 26 million people worldwide, including 6.2 million adults in the United States. With fewer than 6,000 heart transplants performed annually, the need for innovative mechanical circulatory support solutions is more pressing than ever. The National Institutes of Health estimates that up to 100,000 U.S. patients could immediately benefit from advanced heart replacement technology, further underscoring the significance of this breakthrough.
The successful implantation of the BiVACOR TAH highlights The Texas Heart Institute at Baylor College of Medicine and Baylor St. Luke’s Medical Center’s unwavering commitment to revolutionizing cardiac care. By pioneering transformative innovations, we are shaping the future of cardiovascular medicine and delivering new hope to patients worldwide. The future of heart care is here, and we are leading the way.
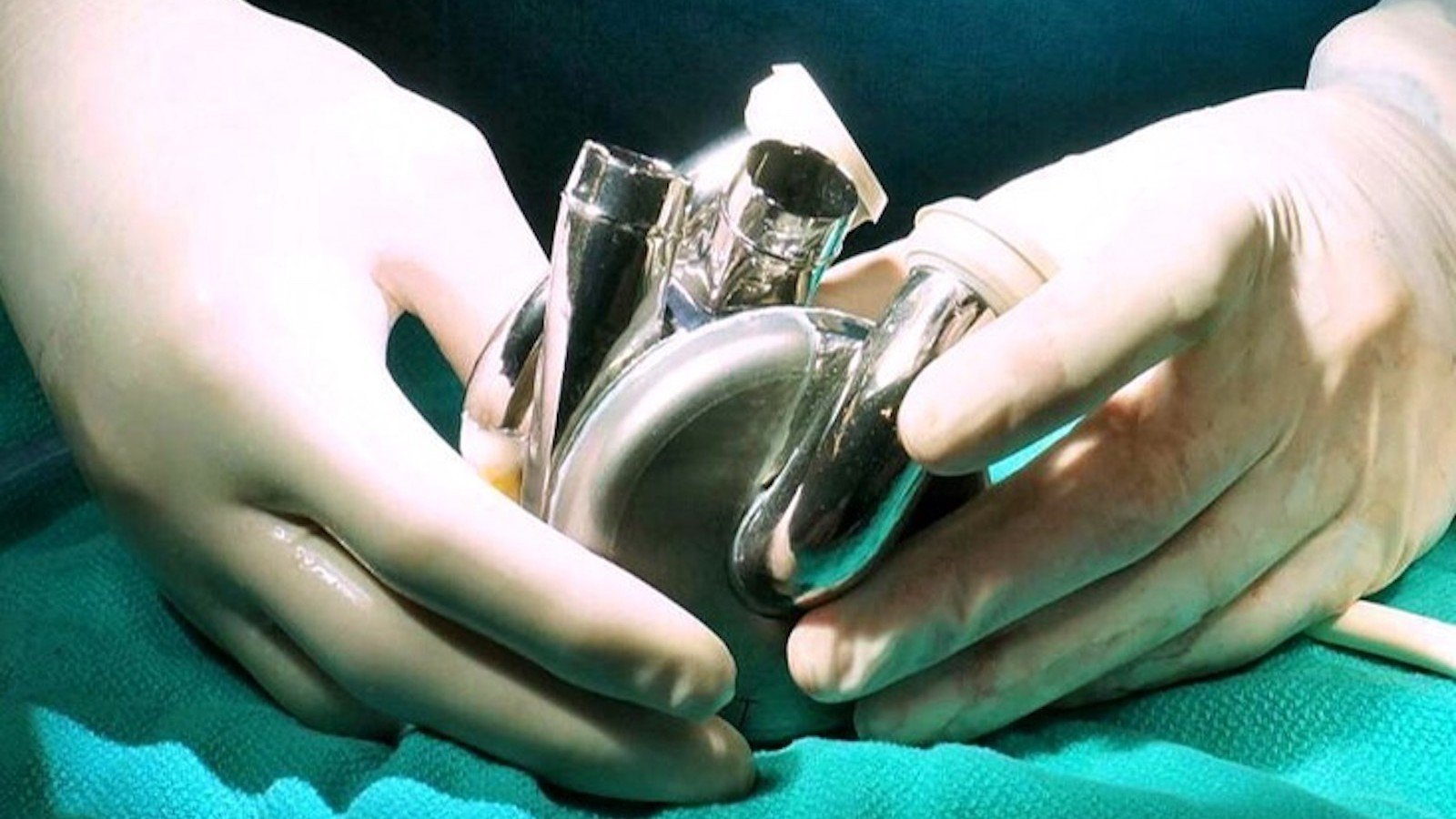
Researchers at The Texas Heart Institute at Baylor College of Medicine are collaborating with three other major institutions on the development of a novel left ventricular assist device (LVAD) as an alternative treatment option to cardiac transplantation and long-term support in end-stage heart failure.
The research being conducted at Baylor St. Luke’s Medical Center, is funded by a four-year, $7.8 million grant awarded to Georgia Tech by the US Department of Defense Congressionally Directed Medical Research Programs (CDMRP) as part of the agency’s call for the development of less invasive treatment technologies for cardiovascular conditions associated with cardiomyopathy.
The goal of this new research is to address several of these shortcomings associated with current LVADs, making LVAD therapy more effective and less invasive. The collaborators propose device design improvements that will reduce blood damage, blood clot formation, and driveline complications such as infection and mobility limitations. The investigators will leverage an interdisciplinary approach involving four research groups with complementary technical expertise and a panel of cardiologists from the U.S. Department of Veterans Affairs. The teams will combine innovative engineering designs, antithrombotic slippery magnetically levitated driving systems and preclinical device testing to achieve their goal.
When completed and evaluated for use in patients through clinical trials and the regulatory approval process, this novel LVAD could provide a much less invasive form of long-term support for patients with heart failure, benefiting the military, veterans, and the public.
The SLIC LVAD also has the potential to greatly improve the quality of life of patients who need circulatory support. Importantly, many of the innovative technologies developed during the project, such as the wireless power transfer for medical devices and the coatings to prevent blood clotting, can be translated into other applications.

A dedicated team of researchers and physicians are bringing transformative innovation to the treatment of congenital heart disease, moving closer to realizing a breakthrough that could save the lives of countless children. A collaborative effort among The Texas Heart Institute at Baylor College of Medicine, Texas Children’s Hospital, and the University of Nevada, Las Vegas (UNLV) has been recognized as one of 14 recipients of the 2024 Single Ventricle Research Fund (SVRF) for their pioneering proposal.
Under the leadership of renowned surgeon Dr. Iki Adachi and a distinguished team including Dr. Nobuyuki Kurita, Dr. Chris Broda, Dr. Yaxin Wang, Dr. Chris Chan, and Dr. Huang Chen, the team is advancing a novel implantable device known as “ReVolution.” This state-of-the-art pump is designed specifically for infants with congenital heart disease who have undergone the Fontan procedure. Unlike conventional methods that provide only temporary circulatory support, the ReVolution pump creates a “biventricular” circulation, making it adaptable to diverse anatomical structures.
What sets the ReVolution apart is its use of cutting-edge maglev centrifugal blood pump technology. This innovative feature facilitates the connection of the vena cava and pulmonary artery, improving blood flow to the left and right pulmonary arteries. The maglev design minimizes shear stress and prevents blood stagnation, significantly reducing risks and enhancing long-term durability.
The potential impact of the ReVolution pump is groundbreaking. By providing a durable, long-term solution, this device could drastically reduce or even eliminate the need for heart transplants among Fontan patients. Not only does this innovation promise to extend life expectancy and enhance quality of life, but it also has the potential to significantly lower healthcare costs associated with managing chronic complications. Furthermore, this breakthrough is expected to drive new frontiers in congenital heart disease research, paving the way for novel treatment strategies for other complex cardiac conditions.
Congenital heart disease (CHD) affects approximately 1 in 100 newborns annually, with single-ventricle heart disease representing one of the most severe forms. The Fontan procedure, a common surgical intervention for these patients, relies on venous pressure alone to drive pulmonary circulation, often leading to complications over time.
The SVRF award program is dedicated to supporting bold, high-impact research that accelerates discoveries and improves care for individuals with single-ventricle heart defects. These highly competitive grants provide up to $600,000 over three years, fueling transformative research that has the potential to redefine the future of congenital heart disease treatment.
The Texas Heart Institute at Baylor College of Medicine and Baylor St. Luke’s Medical Center continue to redefine the field of heart failure treatment successful patient outcomes through groundbreaking advancements in mechanical circulatory support. From the first-in-human implantation of the BiVACOR Total Artificial Heart to the development of next-generation LVADs and the revolutionary ReVolution heart pump for infants, these institutions remain at the forefront of innovation, resulting in more lives saved than ever before. By pioneering novel therapies that enhance patient outcomes and expand treatment options, The Texas Heart Institute and Baylor St. Luke’s are setting the standard for excellence in heart failure care and mechanical support, ensuring the best possible future for patients with advanced cardiac disease.
Patients whose aortic root needs replacing because of an aneurysm can experience better outcomes and improved quality of life, thanks to several options. One of the most promising refinements over the last three decades is a complex technique that spares the heart’s native aortic valve while replacing the surrounding root. Surgeons at The Texas Heart Institute at Baylor College Medicine have perfected this valve-sparing approach, commonly known as the David procedure.
Contemporary approaches to aortic root replacement all have advantages and disadvantages that help inform decision making. Regarding valve-replacing approaches, bioprosthetic valves have limited durability and mechanical valves requires the patient to take blood thinners for life and can cause bleeding. Valve-sparing aortic root replacement (VSARR) avoids these drawbacks and maintains the superior hemodynamic function of the native valve.
VSARR has become the procedure of choice for patients whose aortic valve anatomy is amenable; this commonly includes patients with heritable thoracic aortic disease (such as Marfan syndrome) and patients with coexisting bicuspid aortic valve and root disease. Patients seeking VSARR often site lifestyle as an important consideration. We and others have demonstrated that such patients face low operative risk, even in complex reoperative scenarios.
Understanding the long-term consequences of VSARR is important. At the Institute, our surgeons and clinical researchers have formally studied this approach compared to valve-replacing approaches for over two decades and currently lead a multi-center trial. We found no difference in survival or major complications, with a substantial preference for VSARR in both surgeons and patients.
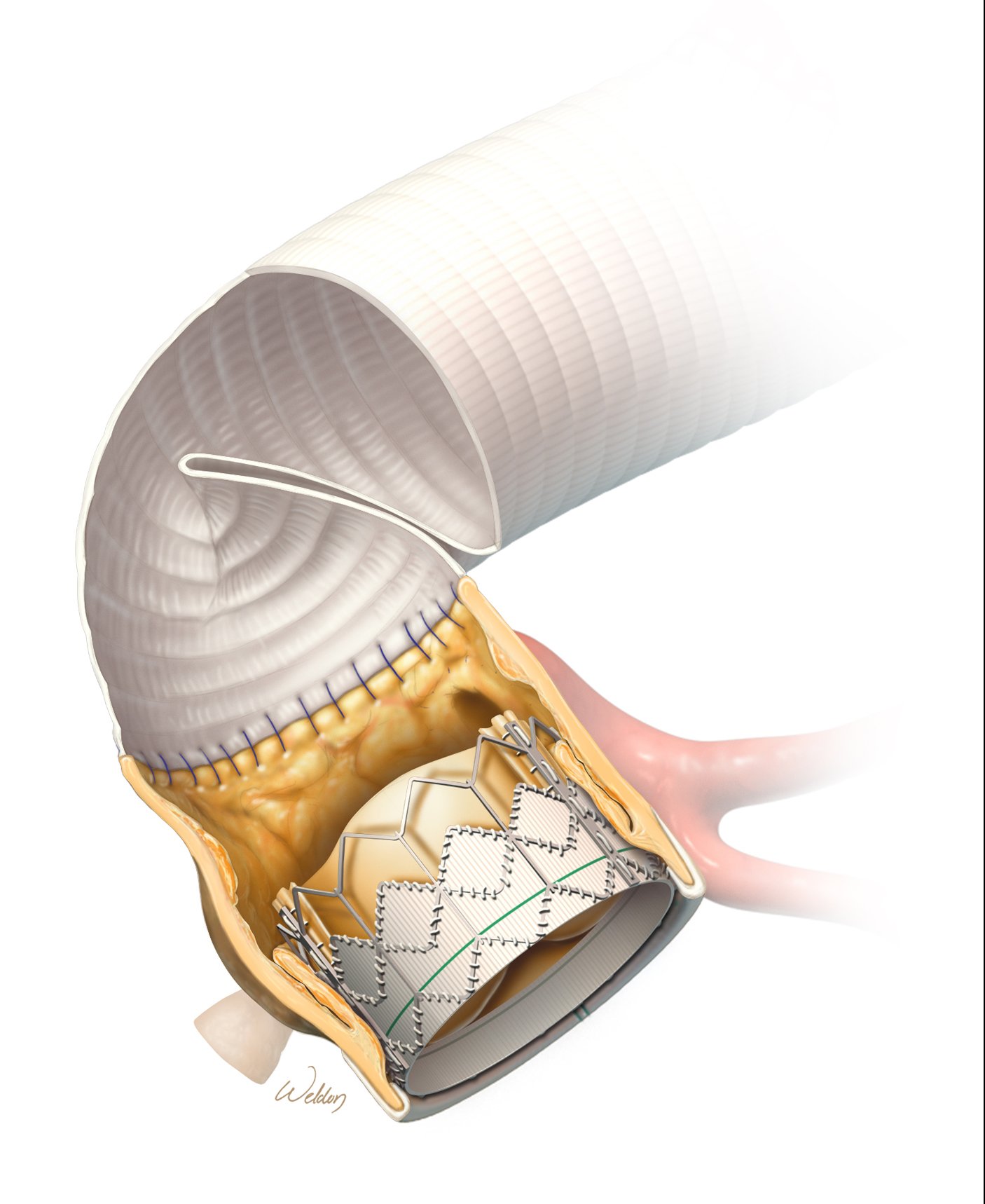
Just like a person’s own aortic valve, bioprosthetic replacement valves can become calcified or stenotic (narrowed) over time, which can prevent the valve from opening completely with each heartbeat. The traditional approach to replacing a defective bioprosthetic relies on redo open heart surgery to remove the old valve and replace it with a new one. However, just like transcatheter aortic valve replacement (TAVR) is disrupting traditional aortic valve replacement by accessing the heart through a blood vessel instead of a large chest incision, TAVR is emerging as an alternate approach to treat poorly functioning, tissue-based prosthetic valves.
Rather than subjecting a patient to another open-chest surgery to replace or repair a dysfunctional bioprosthetic valve, surgeons may be able to place a new transcatheter valve into the previously replaced valve. The technique was described in a recent case report from cardiothoracic physicians at The Texas Heart Institute at Baylor College of Medicine in Houston, Texas.
This so-called “valve-in-valve” approach was used in a 61-year-old patient who, five years previously, had undergone aortic valve replacement with a 25-mm Edwards INTUITY Elite bioprosthetic valve. This valve is made of bovine pericardial tissue to refashion the valve’s leaflets and a metal stent framework that includes a stainless-steel “skirt” for support.
At this patient’s annual checkup, calcium deposits were found on the INTUITY valve’s leaflets, which impede blood flow through the narrowed valve opening. Case discussion by a multidisciplinary care team led to the decision to deploy a transcatheter valve inside the patient’s existing INTUITY valve. This process moves the existing leaflets against the skirt framework, and the leaflets of the new valve take over the valve’s functional role. It is crucial to select a transcatheter valve that maximizes the size of the valve when open.
The new valve was positioned flush with the bottom of the INTUITY valve’s skirt—intentionally lower than is generally recommended—to avoid blocking the coronary arteries, which supply oxygenated blood to the heart itself. Mild leakage around the valve after it was deployed was corrected with careful reshaping. The patient recovered well and was discharged two days later.
Valve-in-valve placement of a transcatheter valve inside an existing bioprosthetic valve (or bioprosthetic root) avoids the injury and complications associated with redo sternotomy and is therefore of great interest to both patients and surgeons.
Recently, our surgeons have even used this valve-in-valve approach to treat a compromised transcatheter valve. The successful use of emerging valve-in-valve techniques rely on having an experienced, multidisciplinary team that is familiar with a multitude of commercially available valves and can navigate the complexity of correctly positioning and sizing the secondary valve without limiting blood flow into or out of the heart to achieve good hemodynamic outcomes and reduce strain across the valve.
A multidisciplinary team at Baylor St. Luke’s Medical Center successfully performed a life-saving heart valve replacement in a 66-year-old patient with alpha-gal syndrome
Alpha-gal syndrome is a rare allergic condition triggered by a sugar found in mammalian tissue, making standard cardiac procedures—many of which involve materials derived from cows or pigs—particularly challenging. The patient, Elton Youngblood, a physician’s assistant with the Veterans Health Administration, had previously received a bovine bioprosthetic aortic valve due to his active lifestyle, which allowed him to avoid blood thinners. However, the valve deteriorated over time, necessitating a replacement.
Upon being diagnosed with alpha-gal syndrome in 2023, Youngblood reached out to several leading cardiac hospitals, seeking a surgical team willing to navigate the complexities of his allergy. Only Baylor St. Luke’s Medical Center agreed to develop a specialized protocol to ensure his safety during surgery.
When Youngblood’s condition worsened in early 2024 following a COVID-19 infection and secondary pneumonia, Baylor St. Luke’s promptly assembled a multidisciplinary team comprising specialists from cardiothoracic surgery, anesthesiology, cardiology, allergy and immunology, internal medicine, nursing, dietary, pharmacy, and hospital leadership to finalize an approach to allow reimplantation of another bovine valve.
A key challenge was the use of heparin, an essential blood thinner derived from animals, which posed a high risk of an allergic reaction. To mitigate this, the team pre-treated Youngblood with steroids and antihistamines for two days prior to surgery, successfully administering heparin during the operation without any complications.
Despite the uncertainty surrounding the long-term durability of a bioprosthetic valve in patients with alpha-gal syndrome, Youngblood is thriving post-surgery, a testament to the expertise and compassionate care of Baylor St. Luke’s Medical Center. His case sets a precedent for future cardiac procedures in patients with rare allergies, reinforcing the institution’s reputation as a leader in innovative, patient-specific surgical solutions.
In 2024, Dr. Kenneth K. Liao, Professor and Chief, Cardiothoracic Transplantation & Circulatory Support at Baylor College of Medicine, achieved a major milestone in robotic cardiac surgery, successfully completing his 700th procedure at Baylor St. Luke’s Medical Center using the da Vinci Robotic Surgical System. As one of the nation’s leading robotic heart surgeons, Dr. Liao has been instrumental in transforming cardiac care at Baylor St. Luke’s Medical Center and The Texas Heart Institute at Baylor College of Medicine, where he has led one of the fastest-growing robotic heart surgery programs in the U.S. since joining in 2019.
Robotic-assisted heart surgery offers unmatched precision and minimally invasive benefits, utilizing a 3D high-definition scope and robotic-controlled instruments that allow Dr. Liao to perform intricate cardiac procedures with exceptional accuracy. Patients experience smaller incisions, less blood loss, reduced pain, lower risk of complications, and faster recovery times compared to traditional open-heart surgery.
Dr. Liao and his team specialize in two primary robotic heart procedures:
With two decades of experience in robotic cardiac surgery, Dr. Liao adopted robotic technology early in its FDA-approved use.
“The magnification provided by the technology offers a tenfold enhancement of our vision, allowing us to discern valve pathology and artery anatomy with unparalleled clarity,” said Dr. Liao. “Additionally, using fine-tipped robotic instruments minimizes tissue trauma, significantly reduces blood loss, and accelerates patient recovery.”
Dr. Liao’s milestone underscores Baylor St. Luke’s Medical Center and The Texas Heart Institute at Baylor College of Medicine’s leadership in cutting-edge, patient-centered cardiac care, ensuring more patients benefit from advanced, minimally invasive surgical techniques.
Through cutting-edge surgical advancements such as valve-sparing aortic root replacement, robotic-assisted cardiac procedures, and innovative solutions for complex cases like alpha-gal syndrome, The Texas Heart Institute at Baylor College of Medicine and Baylor St. Luke’s Medical Center continue to lead the field of cardiac surgery. Their expertise in minimally invasive techniques and personalized surgical interventions ensures that patients will receive world-class care with improved outcomes, faster recovery times, and enhanced quality of life. By integrating the latest technology and surgical precision, these institutions are shaping the future of cardiovascular surgery and reinforcing their status as global leaders in the field.

Kenneth K. Liao, MD, PhD
In early 2025, translational and clinical investigators at The Texas Heart Institute at Baylor College of Medicine will be initiating a first in human clinical trial examining an innovative gene therapy designed to help the heart repair itself after injury. This cutting-edge approach is being explored in collaboration with YAP Therapeutics
In 2024, a landmark study published in Nature Cardiovascular Research provided compelling evidence that the human heart possesses regenerative potential. This research, led by Dr. James F. Martin, Vivian L. Smith Chair in Regenerative Medicine and Vice Chairman and Professor of Molecular Physiology and Biophysics at BCM, utilized single-cell genomics and genetic experiments to uncover fundamental mechanisms of cardiac self-repair.
Challenging long-held beliefs, the study demonstrated that cardiomyocyte regeneration requires a dynamic microenvironment, where a coordinated interplay between cardiomyocytes, resident immune cells, and cardiac fibroblasts drives cardiac renewal. Through intricate signaling pathways, these cells support each other, enabling cardiomyocyte proliferation and effective heart tissue repair.
“These findings challenge the long-held belief that advanced heart failure is irreversible,” said Emerson Perin, MD, Medical Director at The Texas Heart Institute. “This research paves the way for a potential paradigm shift in treatment, giving millions of patients with chronic heart failure hope for a therapy that goes beyond symptom management.”
The implications of these groundbreaking discoveries are profound, offering a glimpse into a future where heart disease may no longer be an irreversible condition. By harnessing the body’s natural regenerative capabilities, this gene therapy has the potential to revolutionize treatment for millions of individuals affected by heart disease, bringing hope for longer, healthier lives.
A groundbreaking clinical trial conducted at Baylor St. Luke’s Medical Center by The Texas Heart Institute at Baylor College of Medicine has demonstrated the potential of cell therapy in treating chronic heart failure exacerbated by inflammation. Led by Emerson Perin, MD, a global authority in regenerative medicine and principal investigator of the DREAM-HF trial, this research represents a pivotal step toward transforming the treatment landscape for heart failure patients.
Published in the December 2024 issue of the European Journal of Heart Failure, the study found that a single intramyocardial injection of mesenchymal precursor cells (MPCs) significantly reduced the rate of cardiovascular death, heart attack, and stroke. Unlike conventional treatments that primarily manage symptoms, this innovative approach directly addresses inflammation in damaged heart tissue, offering a disease-modifying therapy with long-term benefits.
The 30-month DREAM-HF landmark trial is the largest cell therapy study to date in patients with heart failure due to reduced ejection fraction, a condition in which the heart’s ability to pump blood is severely impaired. Patients receiving direct cardiac injections of immunomodulatory MPCs exhibited notable improvements in cardiac function and overall clinical outcomes. By restoring balance between pro- and anti-inflammatory mechanisms in the heart, this therapy provides new hope for high-risk patients who have exhausted traditional treatment options.
These findings reaffirm The Texas Heart Institute at Baylor College of Medicine’s leadership in pioneering novel therapies for cardiovascular disease. Dr. Perin, who has spent two decades advancing cell-based treatments for the heart, emphasized that this milestone study lays the foundation for integrating cell therapy into mainstream heart failure treatment. By addressing the root cause of inflammation, this innovative approach has the potential to reshape the future of cardiovascular care, offering a new standard of treatment for millions of patients worldwide.

A dedicated research team at The Texas Heart Institute at Baylor College of Medicine is pioneering advancements in bioartificial heart technology may one day provide new hope for patients with end-stage heart failure.
Supported by a five-year, $2 million grant from the National Heart, Lung, and Blood Institute of the National Institutes of Health, our scientists and bioengineers are working to develop lab-grown cardiac tissue that could lead to viable bioartificial hearts. This breakthrough aims to address the global shortage of donor organs and expand life-saving treatment options for patients with advanced heart failure.
This innovative approach involves repopulating decellularized heart scaffolds with new heart cells derived from human-induced pluripotent stem cells. However, current technology lacks the advanced bioreactors necessary to provide essential mechanical and electrical stimulation, as well as the precise nutritional support required for a fully functional, human-sized bioartificial heart.
To bridge this gap, the team is developing a benchtop bioreactor system designed to deliver controlled mechanical and electrical stimulation while simulating blood flow through the heart. This bioreactor will be meticulously engineered to prevent contamination and create an optimal environment for tissue growth. To further enhance the viability of bioartificial hearts, researchers are also refining nutritional solutions to ensure adequate oxygen and fuel supply to developing heart cells.
Their research is hoped to advance the technologies that are critical for the maturation and functioning of bioartificial hearts.
Dr. Jennifer Cozart, a distinguished cardiovascular surgeon at The Texas Heart Institute at Baylor College of Medicine, has achieved a historic milestone as the first in Texas to implant the AtriClip FLEX-Mini device—one of the first 10 procedures performed worldwide.
The AtriClip FLEX-Mini is the smallest-profile surgical left atrial appendage (LAA) closure device available, offering enhanced precision and ease of implantation. Secure LAA closure is a critical component in reducing stroke risk for patients with atrial fibrillation, and the FLEX-Mini’s advanced design builds upon the proven technology of the AtriClip to improve visualization and streamline surgical procedures.
“The introduction of the AtriClip FLEX-Mini represents a major step forward in our ability to deliver precise and effective care for patients with cardiovascular disease,” said Dr. Cozart. “Its innovative design enhances surgical efficiency while maintaining the highest standards of safety. I am proud to be part of this advancement and to contribute to the future of heart care here in Texas.”
Dr. Cozart’s successful implantation of the AtriClip FLEX-Mini reinforces The Texas Heart Institute’s and Baylor St. Luke’s Medical Center’s commitment to integrating cutting-edge technologies into clinical practice. This milestone further establishes the institution as a leader in cardiovascular innovation, continually pushing the boundaries of modern heart surgery to improve patient outcomes.
A collaborative, multi-institutional team spearheaded by researchers at Baylor College of Medicine has been awarded a $31 million grant by the Patient-Centered Outcomes Research Institute (PCORI) to determine the safety and effectiveness of commonly used pacing treatments for cardiac resynchronization therapy in people with heart failure.
The research team is led by Dr. Mihail G. Chelu, Associate Professor of Medicine–Cardiology at Baylor College of Medicine, who is the study’s Principal Investigator; Dr. Kenneth A. Ellenbogen, Professor of Cardiology at Virginia Commonwealth University; and Dr. Richard Holubkov, a biostatistician with the University of Utah. The study will focus on the use of conduction system pacing for cardiac resynchronization therapy in patients with heart failure and conduction system disease.
Heart failure affects every aspect of life for an estimated 6.2 million Americans. This debilitating disease not only decreases quality of life but puts stress on patients and their families by imposing a substantial financial burden. Patients with heart failure are also often faced with frequent hospitalizations and an increased risk of premature death.
More than one-third of all patients with heart failure have conduction system disease—a disorder of the heart’s electrical system, which controls the heart’s rhythm and rate of the heartbeat. Cardiac resynchronization therapy treats this conduction system disease by improving cardiac heart function while also increasing the patient’s functional capacity, thereby reducing hospitalizations and prolonging survival. Cardiac resynchronization therapy is the standard treatment in patients with heart failure with reduced ejection fraction and a wide QRS complex. (QRS represents the electrical impulses in the heart as they spread through the ventricles.)
Cardiac resynchronization therapy can be accomplished by one of two strategies: biventricular pacing or conduction system pacing.
Biventricular pacing is the standard of care, improving heart failure in 60% to 70% of patients by pacing simultaneously from two discrete muscle sites: the right ventricular apex and the left lateral wall. On the other hand, conduction system pacing relies on the direct, rapid pacing of conducting cells, either at the His bundle—the part of the heart that carries signals to the atria and ventricles—or the left bundle branch—the branch that distributes the electrical impulses to the left ventricle. This re-establishes activation of the entire arborized conduction system and cardiac muscle in a highly choreographed and efficient manner that closely reproduces the heart’s natural function.
In their study, Drs. Chelu and Ellenbogen will evaluate the safety and effectiveness of these two pacing treatments in a cohort of 2,136 patients enrolled at 65 sites in the U.S. and Canada.
In the initial stages of the study, these investigators have worked with patients, leaders in health care, and research collaborators to identify the outcomes most relevant to patients: quality of life, patient activity, hospitalization for heart failure, and death. They have also collaborated with the Heart Rhythm Society, American Heart Association, American College of Cardiology, the National Hispanic Latino Cardiovascular Collaborative, the Pacemaker Patient Advocacy Group, WomenHeart, and Mended Hearts.
“In 30 to 40 percent of patients with heart failure and conduction system disease, treatment with biventricular pacing does not improve the heart’s function,” said Dr. Chelu, Principal Investigator of the study. “This might be explained by the slower activation of the heart muscle from only two points. Conduction system pacing, however, has the potential to advance the treatment of heart failure. Pacing in the His bundle or left bundle branch leverages the rapid propagation of electrical impulses through the network of the conduction system, leading to more efficient contraction of the left heart. Furthermore, conduction system pacing involves less expensive generators and leads and sometimes fewer leads, as well as a simpler implant procedure than biventricular pacing. We anticipate that conduction system pacing will lower the great burden of health care costs for patients with heart failure.”
Drs. Chelu and Ellenbogen’s study was selected through a highly competitive review process in which patients, caregivers, and other stakeholders joined scientists to evaluate the proposals. The funding is awarded through a PCORI initiative to support large-scale, high-impact comparative effectiveness research trials that are conducted in multiple phases, allowing the testing and refinement of the study approach. Therefore, this study will involve an initial feasibility phase to maximize the likelihood of full trial success. The study was launched on January 1, 2023 and is anticipated to enroll participants in October 2023.
“This study has the potential to fill an important gap in the evidence that is relevant to a range of health care decision makers, helping them better assess their care options,” said PCORI Executive Director Dr. Nakela L. Cook. “We look forward to following the study’s progress and working with Baylor College of Medicine in Houston and Virginia Commonwealth University in Richmond to share its results.”
PCORI is an independent, nonprofit organization authorized by Congress in 2010. Its mission is to fund research that will provide patients, their caregivers, and clinicians with the evidence-based information needed to make better-informed health care decisions. For more information about PCORI’s funding, visit www.pcori.org.

Mihail G. Chelu, MD, PhD
A groundbreaking approach to preventing and managing ventricular arrhythmias is being developed by an interdisciplinary team of researchers at The Texas Heart Institute at Baylor College of Medicine and the University of Texas at Austin. Backed by a four-year, $2.37 million grant from the National Institutes of Health’s National Heart, Lung, and Blood Institute, this pioneering research aims to revolutionize cardiac rhythm management through the use of injectable hydrogel electrodes.
The research team has already demonstrated the feasibility of pacing the heart using the hydrogel in a porcine model, marking a significant step toward developing a less invasive, more effective therapy for patients with ventricular arrhythmias. This innovation was recently featured in Drug Discovery News, which highlighted its potential to provide painless defibrillation, eliminating the distressing shocks associated with current defibrillation techniques.
This breakthrough is the result of a collaboration between Mehdi Razavi, MD, at The Texas Heart Institute, and Elizabeth Cosgriff-Hernandez, PhD, at The University of Texas at Austin. Their work focuses on developing flexible, conductive hydrogel "wires" that could transform cardiac resynchronization therapy. By seamlessly integrating with heart tissue, these hydrogels could provide a novel platform for painless ventricular defibrillation, vastly improving patient outcomes and quality of life.
The integration of hydrogel electrodes into clinical practice has the potential to redefine the treatment landscape for ventricular arrhythmias, offering a less invasive and more patient-friendly alternative to conventional methods. This advancement underscores The Texas Heart Institute’s commitment to pushing the boundaries of innovation in cardiovascular medicine and improving the lives of patients worldwide.
With transformative research in gene therapy, cell therapy for heart failure, and bioartificial heart development, The Texas Heart Institute at Baylor College of Medicine and Baylor St. Luke’s Medical Center are pioneering the future of regenerative cardiovascular medicine. Their work in stimulating heart muscle regeneration and developing innovative treatments for lethal arrhythmias showcases a commitment to shifting the paradigm in heart disease management. By harnessing the power of regenerative medicine, The Texas Heart Institute at Baylor College of Medicine continues to open new frontiers in cardiac care, providing groundbreaking solutions for patients who previously had limited treatment options, and solidifying its reputation as a leader in advancing the next generation of heart therapies.

Recent and ongoing studies at the Center for Cardiometabolic Disease Prevention have tested drugs in development that use RNA-silencing strategies to target lipid disorders associated with cardiometabolic disease and other complications. High triglyceride levels have become an increasing problem with increased prevalence of obesity and diabetes, and very high triglyceride levels are associated with pancreatitis, but current therapies provide modest triglyceride reductions. Two developing drugs tested reduce triglyceride levels by reducing a key protein affecting triglyceride levels—apolipoprotein C-III—but use different approaches. Olezarsen, an antisense oligonucleotide targeting APOC3 messenger RNA (mRNA), reduced triglyceride levels by 43.5% compared with placebo in patients with familial chylomicronemia syndrome in the BALANCE study, which was published in New England Journal of Medicine. Familial chylomicronemia syndrome is a rare genetic disorder characterized by severely elevated triglyceride levels and recurrent pancreatitis. In the BALANCE study, the incidence of acute pancreatitis was approximately 88% lower in olezarsen patients than placebo patients. Plozasiran, a small interfering RNA (siRNA) targeting APOC3, reduced triglyceride levels by 50–62% in patients with mixed hyperlipidemia (combined elevations of cholesterol and triglycerides) compared with placebo in the MUIR study, which was also published in New England Journal of Medicine. Both studies reported acceptable safety data and support further development of these agents to treat the growing number of patients with elevated triglyceride levels. The Center for Cardiometabolic Disease Prevention is now enrolling patients in 2 new studies of plozasiran, MUIR-3 for patients with elevated triglycerides (150–499 mg/dL) and SHASTA-3 for patients with severely elevated triglycerides (≥500 mg/dL).
Ongoing studies at the Center are also testing RNA-silencing agents in development to treat patients with elevated lipoprotein(a) [Lp(a)], an increasingly recognized risk factor for cardiometabolic disease that is not reduced by most currently available lipid-lowering drugs. Both antisense oligonucleotide and siRNA approaches that target LPA mRNA to reduce Lp(a) level are under investigation to determine their effect on adverse cardiovascular events. Both Lp(a)HORIZON, which is testing the antisense oligonucleotide pelacarsen, and OCEAN(a) Outcomes, which is testing the siRNA olpasiran, are in progress in patients with atherosclerotic cardiovascular disease. ACCLAIM-Lp(a) is still enrolling patients with atherosclerotic cardiovascular disease or at high risk for a first adverse cardiovascular event.
Lp(a) level is determined primarily by genetics, and the potential role of treating Lp(a) elevations with specifically targeted agents may have particular clinical significance for cardiovascular disease prevention in certain populations. After the successful completion of the HeartCare genomic medicine project, which was awarded the CommonSpirit Health Physician Enterprise Vision Award in Academic Excellence for advancing the practice of medicine through key research, cardiologists at Baylor St. Luke’s Medical Center and researchers at Baylor College of Medicine’s Human Genome Sequencing Center expanded the HeartCare pilot program to south Texas and genomic sequencing to test for both cardiovascular disease and diabetes. The Houston Study team and the clinicians in south Texas meet weekly via videoconference. Among the early findings in this predominantly Hispanic population, which is understudied in genomic medicine and historically underserved in healthcare, more than a third of individuals tested positive for at least one LPA risk allele. Given the underrepresentation of Hispanic populations in present genomic databases, and a lower diagnostic rate than in the previous HeartCare project, the investigators suggested developing and implementing population-specific LPA risk alleles and polygenic risk scores to improve individual-level risk assessment and management. These results were presented at the American Society of Human Genetics 2023 Annual Meeting in Washington, DC.
The very first coronary care units were established in the early 1960s to help reduce mortality rates among patients with acute myocardial infarction. The main purpose of the CCU was to closely monitor these high-risk patients during the post-infarction period, and specifically to expedite the rapid deployment of external defibrillation and treatment for malignant dysrhythmias in the hope of reducing rates of sudden cardiac death. This required a highly specialized and well-trained intensive care unit team and formed the basis of our current understanding of cardiac critical care.
Over the past few decades, the coronary care unit has gradually undergone significant changes in structure, patient demographics, and overall purpose. With the advent of timely revascularization, clear improvements in the peri-myocardial infarction period were quite evident.
Over 4 million intensive care unit (ICU) admissions occur annually in the U.S., and mortality rates among admitted patients are as high as 20%. This is because, with modern health care, patients are living longer despite having more comorbid conditions. A large proportion of these patients have significant cardiac comorbidities such as heart failure, ischemia, valvular heart disease, pulmonary hypertension, and arrhythmias. Therefore, the Coronary Care Unit has now shifted its focus to a more encompassing Cardiac Intensive Care Unit role.
Changes in patient demographics have been met with a novel approach to training future generations of cardiologists in critical care medicine. Often, patients with significant cardiac comorbidities are admitted to the ICU with non-cardiac primary diagnoses, which requires a highly specialized ICU team to manage all aspects of care.
At the Cardiac Intensive Care Unit (CCU) at Baylor St. Luke’s Medical Center, we strive to continue evolving and adapting to the challenge of providing optimal care to our patients. The CCU forms the backbone and safety net for our patients, who are being provided cutting-edge procedures and care. As early adopters of a cohesive co-management model between our world-class cardiologists and intensivists, we have set the standard for modern-day CCU care. Our unit is staffed by compassionate, dedicated nurses, dual-trained cardiologists with critical care training, and seasoned cardiac intensivists to help support our world-class cardiologists.
Creating this model—which focuses on key quality parameters such as preventing hospital-acquired infections, sedation protocols, expedited weaning and ventilator liberation, and optimizing non-cardiac comorbidities—has helped decrease our mortality index to 0.43.
We continue to develop care pathways for different cohorts of patients. These include our ever-increasing group of structural heart patients who undergo transcatheter heart procedures for acute coronary syndrome and cardiogenic shock. This work further illustrates our commitment to provide the best possible care for our patients.
As part of The Heart Beat series of interviews with Dr. Mehdi Razavi and his team, recent graduate of the Rice University Global Medical Innovations Program, Kunal Shah, MBE, shares details about an entirely new way of treating cardiac arrhythmias and preventing sudden cardiac death.
The research underway in the THI’s Electrophysiology Clinical Research and Innovations (EPCRI) Lab, in partnership with The University of Texas at Austin’s Dr. Elizabeth Cosgriff-Hernandez, is testing the ability of hydrogel electrodes to deliver pulses of electricity to the heart. This paradigm-shifting work caught the attention of science writer Robert F. Service at the American Chemical Society meeting in San Diego.
The novel solution, described simply as “liquid wires,” aims to facilitate painless shocks to failing hearts. The goal is to improve the quality of life for patients living with implantable cardioverter defibrillators (ICDs).
The new therapy in development works by injecting a fluid hydrogel solution into a patient’s cardiac vein. Researchers say this method transforms the vein into a flexible hydrogel electrode and captures more heart tissue than current electrotherapies can touch.
The next episode of The Heart Beat will explore how engineers from Dr. Razavi’s lab are developing a unique catheter-based system suitable to deliver this hydrogel therapy.
